Alkenes and Alkynes hmm
Alkenes and Alkynes hmmif you wonder what it is then to bad don't read this because i don't know either. ... I'm just a random person doing a random blog.... yay.. fun...
OKAY.
alkene is double bone that a carbon form and a alkyne is a triple bond that carbon form basicly that's all...
yea please read other people's blog if you want more information....
okay ALL OF ALKENE AND ALKYNE ARE NOT SATURATED
 sigh I'll probably fail with just that so time to make up some more stuff.
sigh I'll probably fail with just that so time to make up some more stuff.first of all for a double/tri bond it always have to be the lowest number so if there are 5 c and the bond is at from left second it won't be 4 pentene but it would be 2 pentene don't ask why because it life
C= C C C C
the H aren't there because im lazy
so we will start with alkene first. for alkene in another words double bond just change the ending from ane to ene. yes that it. not more. its as easy as 1 2 3. OMG I HAVE A QUESTION A VERY SMART STUDENT ASKS SO IT WOULD BE METHENE? NO because how can you have a double with just 1 carbon = = (i the teacher think the student isn't the smartest)
yea so methene won't work but the rest does... butene ethene propene pentaconten
 okay so naming FIND THE LONGEST CONTINUES CHAIN and then PUT THE DOUBLE BOND AT THE LOWEST NUMBER AND NO 6 IS NOT LOWER THAN 2. - - IF THE NUMBER IS BIGGER THAN THE NUMBER OF CARBON IN THE LONGEST CHAIN SOMETHING IS WRONG!!!!! I RAGEEEEE!! RAWR!! also change the ending to ene that's all everything else is basically the same with alkane. THERE IS CIS AND TRANS STUFF HERE
okay so naming FIND THE LONGEST CONTINUES CHAIN and then PUT THE DOUBLE BOND AT THE LOWEST NUMBER AND NO 6 IS NOT LOWER THAN 2. - - IF THE NUMBER IS BIGGER THAN THE NUMBER OF CARBON IN THE LONGEST CHAIN SOMETHING IS WRONG!!!!! I RAGEEEEE!! RAWR!! also change the ending to ene that's all everything else is basically the same with alkane. THERE IS CIS AND TRANS STUFF HEREH3C CH2 CH2 CH = CH CH3
2-hexene
there example
 |
| this makes absolutely no sense |
as always youtube and some random places
okay she has weird accent lol but it pretty detailed
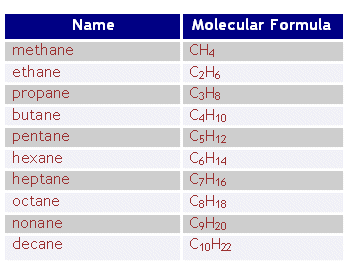
 an alkyl also known as R is an alkane which lost 1 hydrogen. you can find out what is an alkyl group because it is usually the left over parts of the structure. after you find a chain of the carbons, the structure left is the alkyl group.
an alkyl also known as R is an alkane which lost 1 hydrogen. you can find out what is an alkyl group because it is usually the left over parts of the structure. after you find a chain of the carbons, the structure left is the alkyl group.