Today we started to learn about matter. So does matter matter? To be honest no, but for our own good it does. So let’s start.
What is matter? a thing? an object? Well they are all matter because anything that has mass and take up space is consider matter. As I think about the definition of a matter an interesting thing came up to me, so is light considered matter? Short answer yes, long answer is before Einstein came up with his famous equation light isn't matter but after him the definition of matter changed so yes light is also considered matter.
Back to topic, Matter is divided in to 2 sections Pure substance and Mixtures. Pure substance is as its name says "PURE" substance which mean it ONLY contains one kind of particle and of course all the particles have the same properties. Mixtures on the other hand is made out of more than one substance and because it isn't just one substance it have properties that the other substances have.
Pure substance is then divided into two other sections Elements and Compounds. Elements is basically the periodic table, therefore they are the simplest form and can't not be decomposed. Examples are metals, non-metals, and Metalloid. When we put a few elements together, with a bit of energy, and some abracadabra, BOOM they become what we know as compounds. In compound's universe the smallest one of them is called Molecule (I wonder where the word mole theory come from...) of course our favorite molecule is H2O!!!!!
 Mixtures is also divided in two sections. Homogeneous haha... homo... no. Homo as a prefix means same and geneous is a Latin word for genius together they make homogeneous which is a substance that is uniform throughout, it appears to have only one component. Example would be salt water, coffee and other things. The other types appears also to be a geneous. Except it have the exact opposite meaning of homogeneous. Meaning not uniform throughout and appears to have more than one substance. Example would be orange juice with pulp yummm.
Mixtures is also divided in two sections. Homogeneous haha... homo... no. Homo as a prefix means same and geneous is a Latin word for genius together they make homogeneous which is a substance that is uniform throughout, it appears to have only one component. Example would be salt water, coffee and other things. The other types appears also to be a geneous. Except it have the exact opposite meaning of homogeneous. Meaning not uniform throughout and appears to have more than one substance. Example would be orange juice with pulp yummm.Changing matter
Physical change | Chemical change |
NO new substance is formed Chemical composition stays the same reversible | New substance are produced Irreversible Example would be cooking |
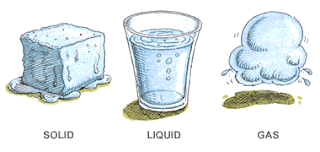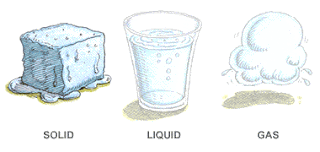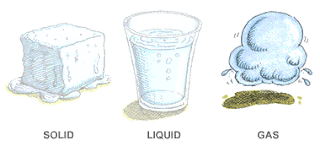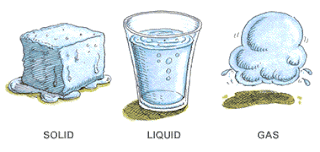
There are 3 states of matter
Solid | Rigid, don’t change shape easily and experience small changes in volume when heated |
Liquid | Takes the shape of the container and experiences slight changes in volume when heated |
gases | Takes the shape of the container and experience drastic changes in volume when heated |

No comments:
Post a Comment