There are many trends within the periodic table whether it is in groups, left right up down or w.e. Here are some of the following
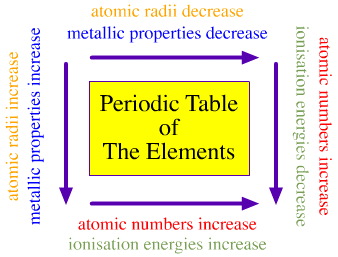
Elements are more metallic as they go down in a family and all the metals are grouped on the left while all the non metals are on the right. The metalloids are in the middle
Atomic radius decreases going right in a period but increases going down a family. Going across a table also increases the atomic number aka protons.
Metals and nonmetals in reactivity is quite funny. When a metal goes down and right once it gets more reactive so if it goes up and left once it is getting less reactive. Nonmetals are opposite of this. Going up in a family increases its reactivity
Electronegativity is a measure of the attraction of an atom for the electron. The higher it is the more attraction it has to electrons. electronegativity decreases as atomic number increases.
Melting and boiling point. The elements near the centre of the table has the highest while noble gases have the lowest
ionization energy is the amount of energy needed to remove 1 electron because it is a noob and not needed. Ion. Energy is increases moving left to right in a period and decreases moving down a family.
No comments:
Post a Comment